5.9 Recall that particles in a liquid have a random motion within a close-packed irregular structure.
5.10 Recall that particles in a solid vibrate about fixed positions within a close-packed regular structure.
October 28, 2011
5.8 Boiling
Understand that a substance can change state from a liquid to gas by the process of evaporation or boiling.
If the temperature of liquid atoms increases even more the particles eventually have enough energy to break the inter-molecular bonds between the atoms, the particles now fly around at very high speeds (several hundred kilometers per hour) and fill any container they are placed in. this is the gaseous state. The state change between a liquid and a gas is called boiling.
If the temperature of liquid atoms increases even more the particles eventually have enough energy to break the inter-molecular bonds between the atoms, the particles now fly around at very high speeds (several hundred kilometers per hour) and fill any container they are placed in. this is the gaseous state. The state change between a liquid and a gas is called boiling.
5.7 Melting
Understand
that a substance can change state from solid to liquid by the process of
melting.
We know that all materials are made of atoms, in a solid state these atoms attract each other and are locked together by the inter-molecular forces between them. But, even in a solid state the atoms are not completely still; they still vibrate on a fixed position.
We know that all materials are made of atoms, in a solid state these atoms attract each other and are locked together by the inter-molecular forces between them. But, even in a solid state the atoms are not completely still; they still vibrate on a fixed position.
If these
atoms are given more internal (kinetic) energy, the particles vibrate faster
and further, if the temperature continues to increase to a point where the
inter-molecular forces between particles are not strong enough to hold the
structure together, but are strong enough to prevent the atoms from flying
apart from each other; a liquid is born. This process of going from a solid
state to a liquid is called melting or fusion.
October 23, 2011
5.6 Pressure Difference
Recall and use the relationship for pressure difference:

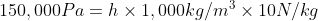
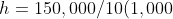)
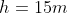


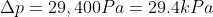
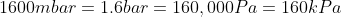
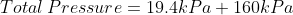
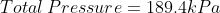
Δp =
pressure of the fluid (N/m2 or Pa)
h = Height of the fluid above loci (depth) (m)
ρ = density of the fluid (kg/m3)
g = gravitational field strength (N/kg)
h = Height of the fluid above loci (depth) (m)
ρ = density of the fluid (kg/m3)
g = gravitational field strength (N/kg)
Proof

- The bottom hole of this column squirts water the furthest
- This is because the water at the bottom has more pressure
- In the formula: Δp = hρg, ρ and g are both constant at all loci, but h is larger lower down \Δp = large.
Questions
The
pressure gauge on a submarine in a river was reading 100kPa when it was the
surface. If a sailor notices that the gauge is now reading 250kPa, how deep is
he? How would the answer change if he were diving in sea water that is slightly
denser that fresh water?
Note: ρfresh water = 1,000kg/m3
Note: ρfresh water = 1,000kg/m3
If the submarine
was in sea water he would be slightly shallower than 15m.
A diver
on Saturn’s moon Titan is 50m below the surface of a lake of liquid methane,
what is the increase in pressure on him due to his depth in the methane? The
density of liquid methane is 0.42g/cm3. The acceleration of gravity
on Titan is 1.4m/s2.
We are told that the pressure of the atmosphere on Titan is 1600mbar. What is the total pressure on the diver (in kPa)?
Note: 1000mbar = 1bar = 100,000Pa.
We are told that the pressure of the atmosphere on Titan is 1600mbar. What is the total pressure on the diver (in kPa)?
Note: 1000mbar = 1bar = 100,000Pa.
5.5 Equal Pressure
Understand
that the pressure at a point in a gas or liquid which is at rest acts equally
in all directions
Magdeburg
Hemispheres
When
the pressure inside the sphere is equal to the atmospheric pressure outside,
the hemispheres can easily be pulled apart because there is no external force
holding the hemispheres together, but if a vacuum is introduced inside the
sphere, the pressure atmospheric pressure pushing against the sphere is much
greater than the pressure pushing outwards, so the resultant force clamps the
sphere closed…
If
you’re confused… I tried my best to explain the pressure as a group of men.
1. No vacuum
There are 50 people inside the sphere trying to push the sphere open and 50 outside trying to keep it shut; obviously the sphere will remain in a stable state as neither side has more strength, but when 2 extra people are introduced (the people holding onto the handles) all of a sudden the side trying to open the sphere has more force than the opposing team. So the sphere comes loose and all the little people can escape.
2.
Vacuum
Inside the vacuum there are no longer 50 people inside trying to break open the sphere, but the 50 people outside (representing the atmosphere) are still there so the sphere remains firmly closed, and when 2 people are introduced to attempt to open the sphere, the sphere remains closed because those two people alone don’t have enough strength to fight against the 50 people working against them.
Inside the vacuum there are no longer 50 people inside trying to break open the sphere, but the 50 people outside (representing the atmosphere) are still there so the sphere remains firmly closed, and when 2 people are introduced to attempt to open the sphere, the sphere remains closed because those two people alone don’t have enough strength to fight against the 50 people working against them.
5.4 Pressure
Recall and use the relationship between pressure, force and area:
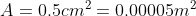
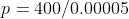
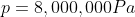
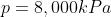
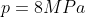
Clearly one can see that the high-heeled show will do the most damage to the floor.
NB: 1N/m2
= 1Pa
Using the
Formula
Calculate
the pressure generated by an ordinary shoe heel (person of mass 40kg, heel 5cm
x 5cm), an elephant (of mass 500kg, foot of 20cm diameter) and a high-heeled
shoe (person of mass 40kg, heel area 0.5cm2). Which ones will
damage a wooden floor that starts to yield at a pressure of 4000 kPa?
1. Ordinary shoe heel
2. Elephant
Clearly one can see that the high-heeled show will do the most damage to the floor.
Subscribe to:
Posts (Atom)


