5.19 use the relationship
between the pressure and volume of a fixed mass of gas at constant temperature:
p1 =
Pressure at the beginning
V1 = Volume at the beginning
p2 = Pressure at the end
V2 = Volume at the end
V1 = Volume at the beginning
p2 = Pressure at the end
V2 = Volume at the end
NB: Can use any units for V and p as long as they are constant at the
beginning and end.
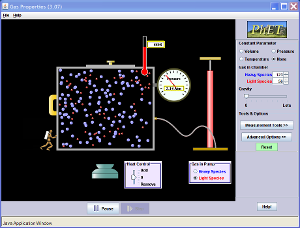
Watch the video below:
We can
clearly see that as we reduce the pressure in the vacuum the volume of the gas
increases significantly, also note that the Volume is constant and the mass of
the gas is constant.
While we’re on the topic of a
vacuum, you might like to take a look at the video below “Nothing” by Vsauce a great YouTube channel for quirky science videos: