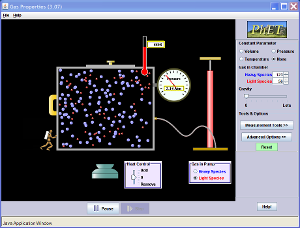
Increasing the temperature of particles in a sealed container causes them to move with larger amounts of kinetic energy so collide with the walls more often and at higher speeds, this means that when the particles come into contact with the container they apply a larger amount of force onto the area and therefore the pressure increases
Increase
in temperature results in an increase in pressure (if volume is constant)
Example:
Cloud formation
- Place a little water in the bottom of a 1½ litre plastic bottle
- Squeeze a few times
- Introduce a small amount of smoke
- Squeeze and release several times
- When you squeeze, the cloud disappears; when you release the cloud reforms.
Explanation
- When the pressure increases the temperature increases and vice versa.
- The smoke particles are nucleating sites on which the water can condense
No comments:
Post a Comment